
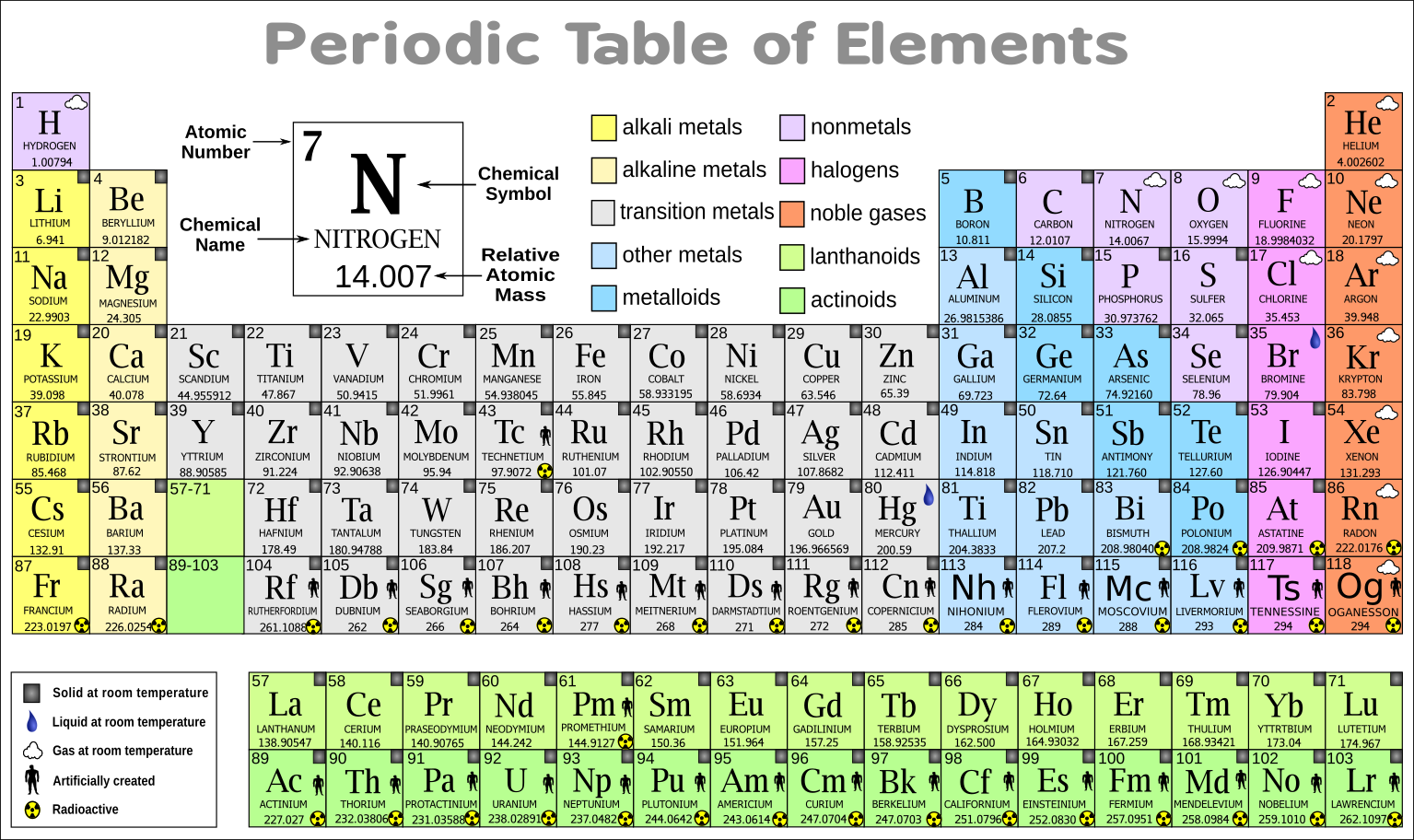
Click on 'Development of the periodic table' to learn about the scientists involved in the table's creation. Explore this collection of videos on each element in the periodic table. Sodium is the fourth most abundant element on earth, comprising about 2.6 of the earth's crust it is the most abundant of the alkali group of metals. The D lines of sodium are among the most prominent in the solar spectrum. The original periodic table emphasized the reactivity of the elements. The modern periodic table emphasizes the electronic structure of atoms. This would mean that indium’s atomic mass was actually 113, placing the element between two other metals, cadmium, and tin. Hover over an element to find out about its discovery and click on it for more information. Sodium is present in fair abundance in the sun and stars. 6.1: Early History of the Periodic Table. Because elemental indium is a silvery-white metal, however, Mendeleev postulated that the stoichiometry of its oxide was really In 2O 3 rather than InO. If this atomic mass were correct, then indium would have to be placed in the middle of the nonmetals, between arsenic (atomic mass 75) and selenium (atomic mass 78). The atomic mass of indium had originally been reported as 75.6, based on an assumed stoichiometry of InO for its oxide. For example, most noble gases have names ending with -on, while most. The names of some elements indicate their element group. If there is a second letter, it is lowercase. Each element has a symbol, which is one or two letters. He discovered, for example, that the atomic masses previously reported for beryllium, indium, and uranium were incorrect. The periodic table lists the elements in order of increasing atomic number. When the chemical properties of an element suggested that it might have been assigned the wrong place in earlier tables, Mendeleev carefully reexamined its atomic mass. The observed properties of gallium and germanium matched those of eka-aluminum and eka-silicon so well that once they were discovered, Mendeleev’s periodic table rapidly gained acceptance. The modern table is derived from Mendeleev's periodic table, but with. The Russian scientist Dmitri Mendeleev is most often credited with inventing the periodic table (1869).

Two of the blanks Mendeleev had left in his original table were below aluminum and silicon, awaiting the discovery of two as-yet-unknown elements, eka-aluminum and eka-silicon (from the Sanskrit eka, meaning “one,” as in “one beyond aluminum”). The periodic table is a tabular arrangement of the chemical elements by increasing atomic number which displays the elements so that one may see trends in their properties. Unfortunately, there was a slightly different system in place in Europe.\)). The first two groups are 1A and 2A, while the last six groups are 3A through 8A. The traditional system used in the United States involves the use of the letters A and B. There are two different numbering systems that are commonly used to designate groups, and you should be familiar with both. These two rows are pulled out in order to make the table itself fit more easily onto a single page.Ī group is a vertical column of the periodic table, based on the organization of the outer shell electrons. 1850-1899 (+23 elements): the age of Classifying Elements received an impulse from the. 1800-1849 (+22 elements): impulse from Scientific Revolution and Atomic theory and Industrial Revolution.

Before 1800 (36 elements): discoveries during and before the Age of Enlightenment. The Periodic Table can be used to determine whether an element is a metal or non-metal Wales. Nevertheless the conventional long form with the 118 group. Figure 2 2: Periodic Table showing when each element was discovered. The usual presentation of the periodic table which we use (figure 1) is subject to continual updating as new elements are produced (Holden and Coplen, 2004). Periods 6 and 7 have 32 elements, because the two bottom rows that are separate from the rest of the table belong to those periods. However, the only specific recommendation IUPAC has made concerning the periodic table covers the Group numbering of 118. Period 1 has only two elements (hydrogen and helium), while periods 2 and 3 have 8 elements. A new period begins when a new principal energy level begins filling with electrons. By initially filling the periodic table in by group you will learn to associate the elements of each group together. You will be surprised at how quickly you can memorize the whole periodic table. There are seven periods in the periodic table, with each one beginning at the far left. You could print out a blank periodic tables and fill them in one column (group) at a time. \) (Credit: User:Cepheus/Wikimedia Commons Source: (opens in new window) License: Public Domain)Ī period is a horizontal row of the periodic table.


 0 kommentar(er)
0 kommentar(er)
